Description
All healthcare workers should follow the standard and transmission-based precautions as described in the Australian Guidelines for the Prevention and Control of Infection in Healthcare (2021)
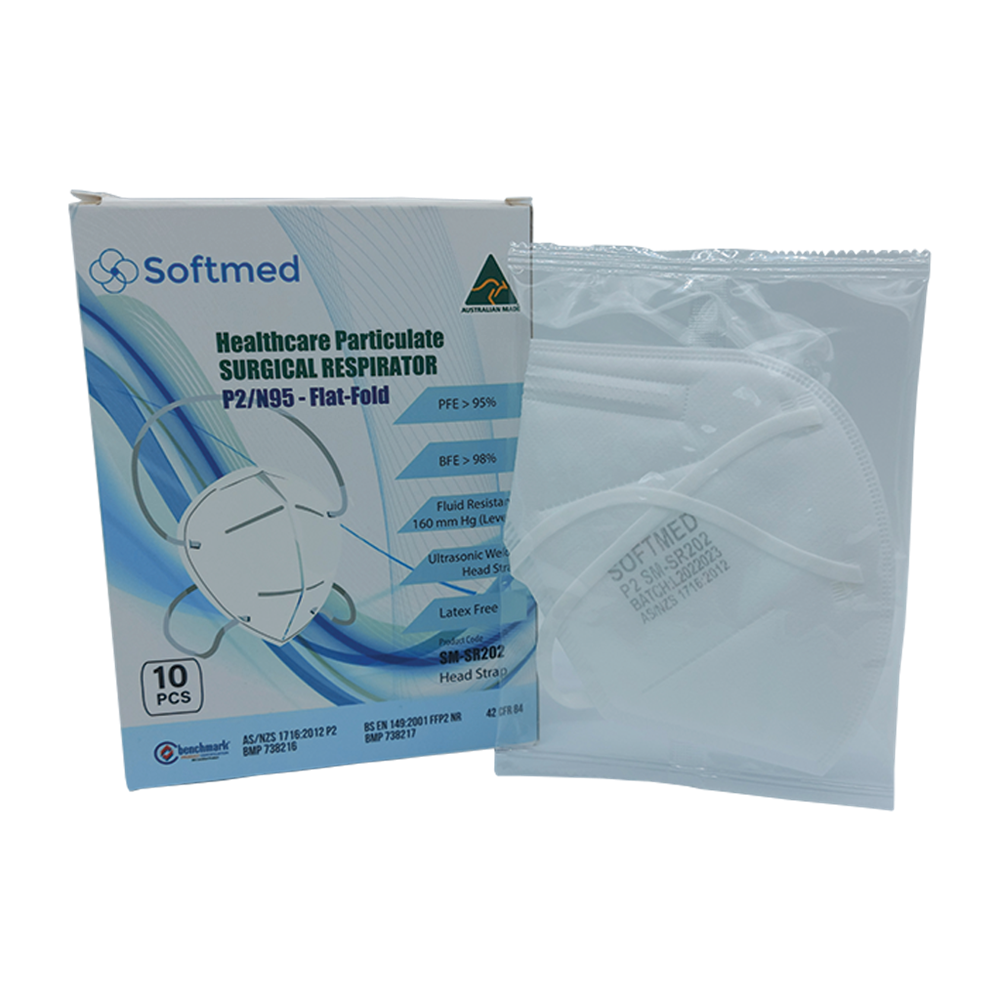
P2/N95 Flat Fold – Healthcare Particulate Surgical Respirator with Head Strap Fluid Resistance – Level 3. P2/N95 Flat Fold Respirator with head straps is intended for single use by operating room personnel and other general healthcare workers to protect both patients and healthcare workers against the transfer of microorganisms, blood, body fluids, and airborne particulates.
5 PLY, P2/N95 Flat Fold - Healthcare Particulate Surgical Respirator with head strap includes 4 layers of non-woven cloth construction that over clinicians’ comfort, protection, and breathability.
P2/N95 Flat Fold Respirator with head straps is suitable for the general public, clinicians during surgical procedures, and front-line workers battling infectious diseases.
5-ply folded respirator is designed with 2-middle melt-blown layers that are critical, as they filter to contain droplets by electrostatically adsorbing them on the surface hence it cannot infiltrate the respirator. While the inner layer is intended to absorb water, sweat, and spit. While the outer layer provides resistance against blood penetration.
P2/N95 Flat fold respirator with head straps is provided in a non-sterile state.
P2/N95 folded respirator is a four-layered respirator made from the following materials:
Spunbond 260mm wide and 50gsm
Cotton 260mm wide and 40gsm
Meltblown 260mm wide and 25gsm
Meltblown 260mm wide and 25gsm
Spunbond 260mm wide and 25gsm
Nose Clip
Head Strap
Softmed Flat Fold Respirators comply with the following Australian and International Standards:
AS 4381:2015 Single-use face masks for use in health care
BS EN 14683:2019
AS/NZS 1716: 2012 Respiratory protective devices
BS EN 149: 2001 Respiratory Protective Devices – Filtering half masks to protect against Particles Requirements, Testing, Marking.
42 CFR Part 84 Respiratory Protective Devices
The Softmed Face Mask N95 Flat Fold Head Strap Box 10

Folded Smart. Fitted Right.
What our customers Say